This fact box will help you to weigh the benefits and harms of prostate cancer early detection using a PSA test. The information and numbers in this fact box represent no final evaluation. They are based on the best scientific evidence currently available.
This fact box was developed by the Harding Center for Risk Literacy.
Prostate cancer is a malignant cell mutation of the prostate gland (prostate) in men. The prostate is one of the man's internal sexual organs and produces a component of seminal fluid. It is located between the bladder and pelvic floor muscles, and surrounds the urethra [2].
Uncontrolled cell growth in cancer can lead to an enlargement of the prostate and an impairment of the urethra, which can cause difficulties in urination (e.g. increased urge to urinate or weaker urine stream). However, prostate cancer does not always cause symptoms or pain. Even benign changes can lead to an impairment of the lower urinary tract [2].
Prostate cancer is the most frequent cancer illness and the third most frequent cancer cause of death in men at an advanced age in Germany. In addition to genetic risk factors (e.g. black skin color and family history), lifestyle-related factors (e.g. obesity, diet) also appear to have an influence on the development of the disease [3].
Early detection tests (also called screening) are aimed at people who show no symptoms related to the disease they are tested for, in this case prostate cancer.
PSA test
The determination of PSA levels in blood by PSA testing is intended to detect and treat prostate cancer at an early stage [2].
PSA stands for prostate specific antigen, a protein produced in the prostate and released into the seminal fluid. Part of the PSA is also released into the blood and can be detected by a PSA test. High PSA levels can mean cell growth in the prostate, but they can also have other causes. For example, an increased level can be caused by tissue changes or irritation (e.g. urinary tract infection or ejaculation) [2].
The PSA test is not part of the cancer early detection program of the German statutory health insurance. It is an individual health service (German: IGeL), the costs of which men have to cover themselves [2].
Men aged 45 years and older who have an estimated life expectancy of more than ten years should be informed about the possibility of prostate cancer early detection by PSA testing. Men with an increased risk of prostate cancer can be informed about the screening five years earlier [4].
In Germany, the digital rectal examination (palpation of the prostate) is currently paid for by the statutory health insurance as an early detection examination. During the palpation examination, the prostate is palpated with a finger through the rectum to assess its size, firmness and surface area. However, there is no evidence that men who regularly participate in the palpation test die less frequently from prostate cancer [2].
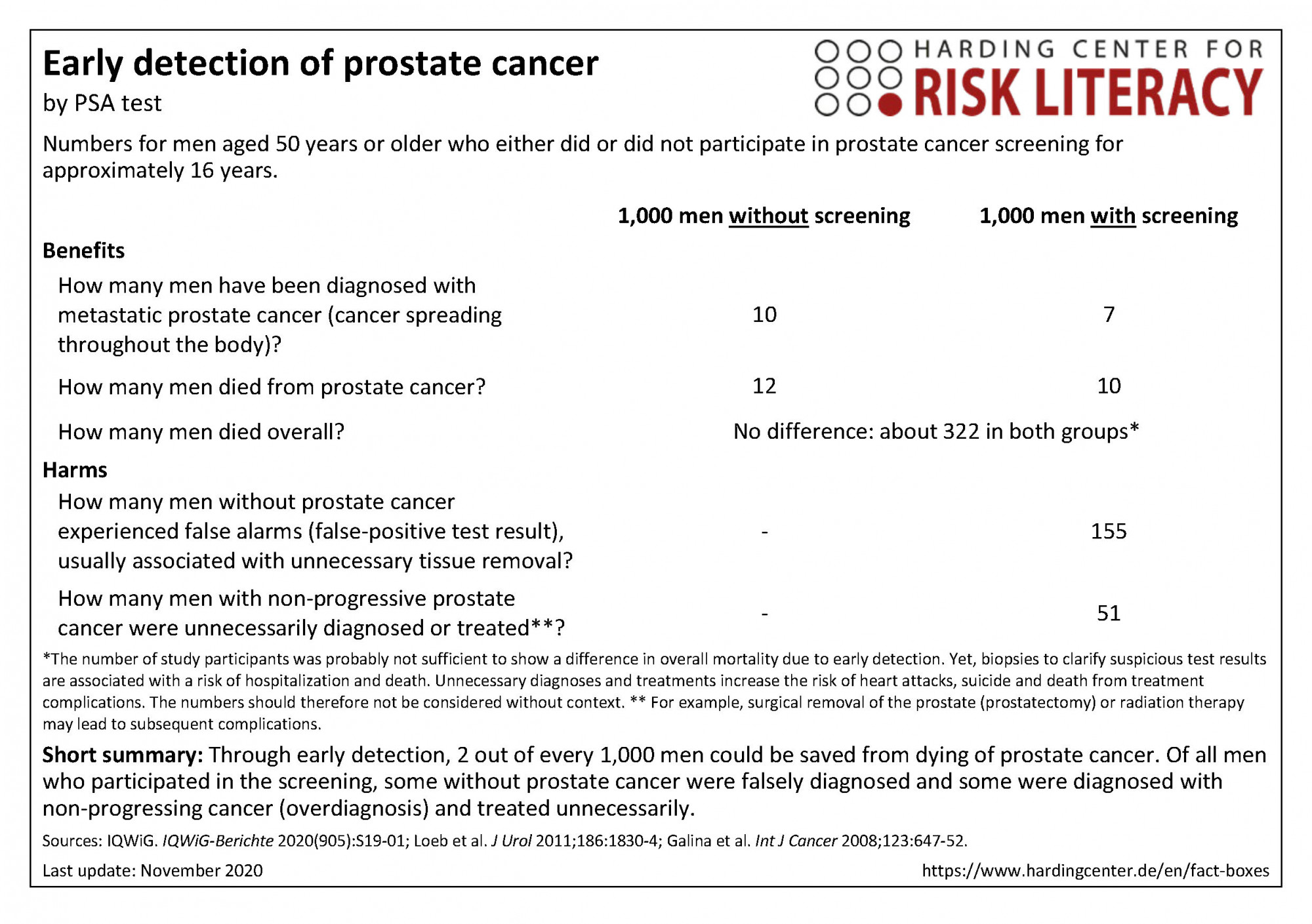
The fact box compares the participation in prostate cancer screening and non-participation regarding benefits and harms.
The table may be read as follows:
About 10 out of every 1,000 men with screening, and 12 out of every 1,000 men without screening died from prostate cancer within 16 years. This means that 2 out of every 1,000 people could be saved from death from prostate cancer by early detection using PSA testing. This was not reflected in overall mortality.
The numbers in the fact box are rounded. The numbers for benefits are based on four studies with about 77,000 participants (progressive cancer), four studies with about 472,000 participants (overall mortality), and eleven studies with about 619,000 participants (prostate cancer specific mortality). The numbers for harms are based on seven studies with approximately 128,000 participants (false-positive results within three to six participations in PSA testing for early detection) and nine studies with approximately 274,000 participants (overdiagnosis and overtreatment) [1].
| Alter unter 50 Jahren | Alter von 50-75 Jahren | Alter ab 76 Jahren | Risikogruppen | |
| Frauen | - | - | - | - |
| Männer | - | X | - | - |
Erklärung der Symbole: X = für diese Personen gelten die Zahlen in der Faktenbox; (X) = auf diese Personen lassen sich die Zahlen unter Vorbehalt anwenden (in solchen Fällen ist eine Rücksprache mit ärztlichem Personal empfehlenswert); - = für diese Personen gelten die Zahlen nicht; ? = es ist unbekannt, ob die Zahlen für diese Personen gelten
Due to the fact that in two of the largest studies numerous men in the control group also had PSA tests performed (contamination), it is unclear whether a PSA cut-off value of 4 ng/ml and higher is actually the characteristic that accounts for the difference between the subgroups [1]. Therefore, it cannot be ruled out that the actual effect of the screening may have been slightly greater.
Overdiagnosis and overtreatment
PSA screening cannot distinguish between non-progressive or slow-growing and progressive prostate cancer. However, prostate cancer is often either non-progressive or grows so slowly that it would not have caused symptoms in many men throughout their lives. Therefore, it is important to weigh the gain in years of life against the loss of quality of life due to the possible adverse effects of treatment (e.g. urinary incontinence, risk of heart attack, suicide or death from treatment complications) [4, 5, 6].
Measures to reduce screening complications
The updated S3 guideline for prostate cancer screening [5] recommends a risk-adapted approach to reduce the risk of screening complications. It recommends regular follow-up examinations at the following intervals, depending on the patient's age and current PSA level:
PSA under 1 ng/ml: interval every 4 years
PSA 1 to 2 ng/ml: interval every 2 years
PSA above 2 ng/ml: interval every year [4].
If a PSA value is increased or at the limit value, it should first be checked under consideration of influencing factors. Only in the case of a significant increase or if the limit value is repeatedly exceeded (PSA value of ≥ 4 ng/ml), a tissue removal (biopsy) should be recommended [5]. The recently changed procedure in Germany (initial examination of the PSA level and regular review as well as wait-and-see observation in case of elevated PSA levels) is intended to prevent early detection from increasing the number of diagnoses (overdiagnosis) and leading to unnecessary treatment (overtreatment). However, it is currently unclear whether and to what extent this approach has an impact on the benefit-harm ratio of prostate cancer screening [1].
Results on overall mortality
No definite statement can be made about the influence of the PSA test on overall mortality. It is therefore not yet certain whether fewer people who have a PSA test repeated within 16 years die. Clinical studies with a total of 500,000 participants were not sufficient to prove a difference in overall mortality. The probability of detecting a difference in overall mortality (if it exists) on the basis of these studies is low. The presumed number of participants required for this is well over 1 million. For this reason, the results on overall mortality alone are not suitable for assessing the benefits of PSA screening.
The evidence was determined by the authors of the included review paper. According to their evaluation, the evidence is of low to high quality, depending on the benefit or harm considered. It is very likely that the results on overall mortality will be changed by further research (low evidence quality).
It is unlikely that the results on the outcomes of progressive prostate cancer, death from prostate cancer, false-positive test results, and overdiagnoses as well as overtreatment will be changed by further research (high evidence quality).
Information for this fact box were obtained from the following sources:
[1] IQWiG. Prostatakrebsscreening mittels PSA-Test. Abschlussbericht S19-01. IQWiG-Berichte 2020:905. Abrufbar unter: https://www.iqwig.de/de/projekte- ergebnisse/projekte/nichtmedikamentoese-verfahren/s- projekte/s19-01-prostatakarzinom-screening-mittels-psa- test.11857.html (30.09.2020)
[2] IQWiG. Gesundheitsinformation zum Thema „Örtlich begrenzter Prostatakrebs“ 2020. Abrufbar unter: www.gesundheitsinformation.de/oertlich-begrenzter- prostatakrebs.2066.de.html#frueherkennung (01.10.2020).
[3] Robert Koch-Institut. Bericht zum Krebsgeschehen in Deutschland 2016. Zentrum für Krebsregisterdaten im RKI 2016:97-8, Berlin Retrieved from: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Kr ebsgeschehen/Krebsgeschehen_node.html (06.10.2020).
[4] Leitlinienprogramm Onkologie der Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, Deutschen Krebsgesellschaft, Deutschen Krebshilfe. Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms 2019: 5.1. Retrieved from: https://www.leitlinienprogramm- onkologie.de/leitlinien/prostatakarzinom/ (13.11.2020).
[5] Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM (2011). Complications after prostate biopsy: data from SEER-Medicare. The Journal of Urology, 186(5), 1830-1834. doi: 10.1016/j.juro.2011.06.057.
[6] Gallina A, Suardi N, Montorsi F, Capitanio U, Jeldres C, Saad F, Péloquin F et al. (2008). Mortality at 120 days after prostatic biopsy: a population‐based study of 22,175 men. International Journal of Cancer, 123(3), 647-652. doi: 10.1002/ijc.23559.
Documentation on how the numbers in the fact box were determined is available on request.
-
November 2020 (update of the research, new evidence; update of the accompanying text)
-
November 2017 (update of the research, no new evidence; update of the accompanying text)
-
Februar 2014 (development)
