This fact box will help you weigh the benefits and harms of non-invasive prenatal testing for the detection of specific genetic disorders (trisomies). The information and numbers in this fact box represent no final evaluation. They are based on the best scientific evidence currently available.
This fact box was developed by the Harding Center for Risk Literacy.
In the nucleus of each cell, genetic information about a human being is stored in the so-called chromosomes. Every human being has 46 chromosomes.
In very rare cases, genetic disorders can occur during cell division. In some cases, a chromosome is present three times. This means that the offspring has a trisomy, which is called differently depending on the chromosomes affected (e.g. trisomy 21, when the 21st chromosome appears three times instead of twice) [1, 2].
The severity and clinical implications are different for different trisomies [1].
- Trisomy 21 occurs in about 140 to 230 out of every 100,000 offspring.
- Trisomy 18 occurs in about 59 out of every 100,000 offspring.
- Trisomy 13 occurs in about 23 out of every 100,000 offspring.
The probability of these and other genetic disorders increases with the mother’s age.
The NIPT is used for the early detection of genetic disorders in pregnancy. It is a supplementary diagnostic tool in case of an abnormal combined first trimester screening (which is another blood test of the mother and an ultrasound examination of the fetal neck area, the so-called nuchal translucency scan). The NIPT can be performed from the 10th week onwards of pregnancy. For this purpose, a blood sample is taken from the pregnant woman, because in addition to genetic information (DNA) from the mother, parts of the offspring's DNA (cffDNA) can also be found there. These parts of the offspring's genetic material usually come from the placenta. During the test, they are filtered and sorted from the mother's blood. For example, if an excessive number of parts of chromosomes 21, 18 or 13 are found, this can be used to determine trisomy 21, 18 or 13 [3].
The examination of the blood sample takes up to two weeks. The result is not a final diagnosis, but only an estimation of the existing risk of trisomy in the offspring. Three different findings are possible and are indicated separately for each genetic change: "low risk", "high risk" and, for some tests, also "unclear risk" [3]:
"Low risk": A genetic disorder can be ruled out by nearly 100 percent.
"High risk": In mothers from this risk group, the offspring has a genetic disorder in nine out of ten cases.
"Unclear risk": The test does not provide a useful result. This can be caused by a sample with too little fetal DNA. It can also occur if the mother is overweight. The test can then be repeated with a new blood sample.
Before taking the blood sample and after the results are available, the doctor is required to provide detailed counseling to the pregnant woman. In addition, doctors need to obtain written consent to document that the mother really wants to know the test results [2].
This fact box considers two different procedures for NIPT, which are presented in a combined format:
Massively parallel shotgun sequencing (MPSS)
In this procedure, all DNA parts of the maternal blood sample are randomly analyzed [1].
Targeted massively parallel sequencing (TMPS)
In this procedure, the maternal blood is analyzed only for specific DNA parts [1].
These methods may have different trade names depending on the test provider (e.g., Harmony Prenatal Test®, PraenaTest® or Panorama Test®).
It is possible that the NIPT does not detect an existing genetic disorder (false-negative result). Some rare forms of trisomies 21, 18 and 13 cannot be reliably detected by a blood test, for instance, if only part of the child's somatic cells have the corresponding chromosome three times, while other cells have a regular set of chromosomes (so-called mosaic trisomy) [1].
False-positive results (false alarms) are also possible. This means that a high risk may be detected although there is no genetic disorder in the offspring. For this reason, a so-called invasive examination, by means of an amniocentesis or placental punctures (chorionic villus sampling), is always recommended by the physician in order to refine the test result in the case of an abnormal finding [2]. This involves using a fine needle to remove some amniotic fluid from the amniotic sac or some tissue from the placenta. Both types of examination carry a risk: a miscarriage occurs in about 1 to 2 women out of every 100 as a result [1].
The following figures show the frequency of false-negative and false-positive results in the TMPS procedure after first trimester screening for women with an average age of 25 years, 35 years and 40 years respectively. It is assumed that, based on 100,000 women in each age group, 81 women aged 25 years (0.08%), 300 women aged 35 years (0.3%), and 1,020 women aged 40 years (1%) are at high risk for indication of a genetic disorder [4].
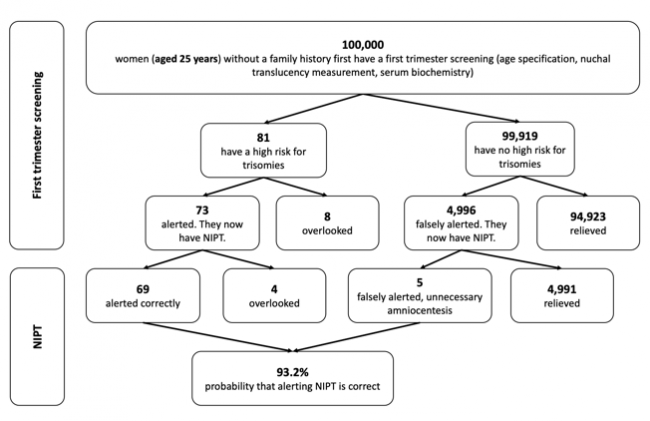
Figure 1: Reliability of NIPT (TMPS) following first trimester screening to detect high risk of trisomy 21 (Down syndrome) in pregnancy at an average age of 25 years
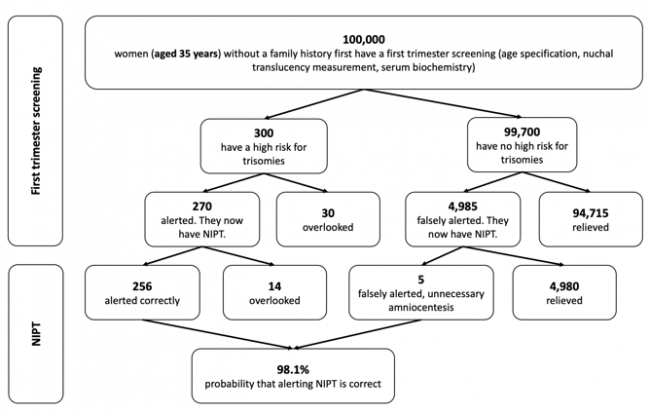
Figure 2: Reliability of NIPT (TMPS) following first trimester screening to detect high risk of trisomy 21 (Down syndrome) in pregnancy at an average age of 35 years
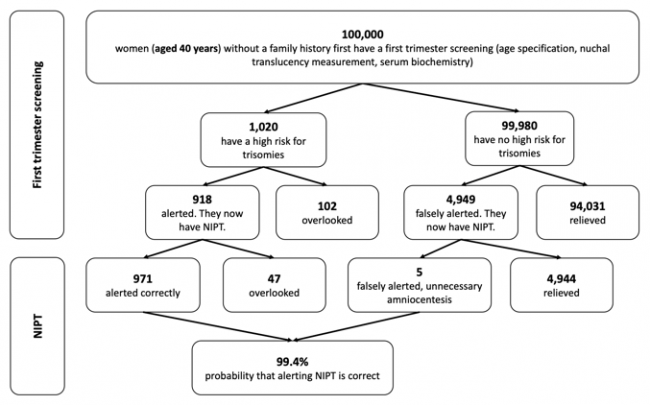
Figure 3: Reliability of NIPT (TMPS) following first trimester screening to detect high risk of trisomy 21 (Down syndrome) in pregnancy at an average age of 40 years
Pregnant women of any age might consider NIPT following the first trimester screening. It is offered for both normal and high-risk pregnancies, but can only be billed by the statutory health insurance under certain conditions [1, 3].
An alternative option is not to participate in any prenatal screening (first trimester screening, NIPT). Some pregnant women prefer to experience their pregnancy in "good hope" and wait observantly, while others prefer to know as much as possible in advance to consider preparations or abortion.
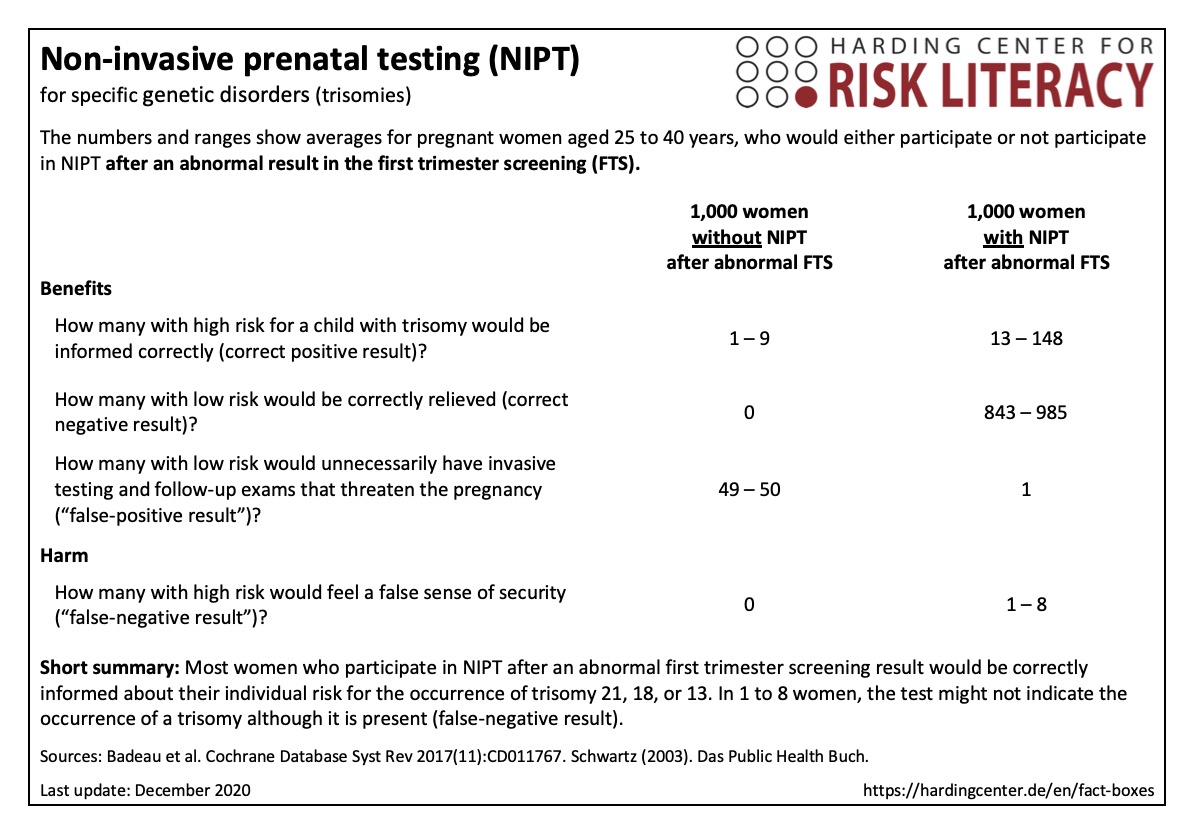
The fact box assesses the use of NIPT, based on its ability to correctly predict the presence of trisomy 13, 18, or 21.
The table may be read as follows:
843 to 985 out of every 1,000 women, who have an NIPT due to an abnormal first trimester screening result, would be correctly informed by a negative NIPT result. In 1 out of every 1,000 women NIPT would falsely indicate a trisomy (false-positive test) and result in unnecessary follow-up examinations. In 1 to 8 women, the test might not indicate a trisomy, although it is present (false-negative result).
The numbers in the fact box are rounded. They are based on 65 studies with about 86,000 participants [1].
The participation in prenatal screenings (first trimester screening, NIPT) is a decision that should be based on the personal preferences and expectations of the expecting mothers and her relatives. No doctor, obstetrician, or other medical professional should force her to make a decision [3].
In general, the NIPT should not be regarded as a stand-alone diagnostic tool. In Germany, the NIPT is used if abnormalities or ambiguities occur during the first trimester (following combined first trimester screening). Starting in the 11th week of pregnancy, the fetus in the mother's womb is examined by a physician. This includes an anatomical assessment of the fetus by ultrasound examination, measurement of nuchal fold transparency, and various blood tests, which already allow to reliably rule out many malformations [3].
All decisions that an expectant mother has to make should also take into account that, for example, the care of children with trisomy 21 and the counseling options for their families have changed fundamentally over the past decades. These changes, including medical and surgical advances, specific health promoting interventions, and support for parents and family members, have helped people with trisomy 21 live longer and experience a better quality of life. Their median life expectancy today is 58 years. People with trisomy 21 contribute to the diversity of a society. No one can make predictions about the future or development of a child with trisomy 21, but even without trisomy 21, such predictions are not possible [1].
The evidence was assessed by the authors of the review included. According to their assessment, the evidence is of overall moderate quality.
Further research might change some of the results.
- December 2020 (development)
Information within the fact box was obtained from the following sources:
[1] Badeau M, Lindsay C, Blais J, et al. Genomics‐based non‐invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database of Systematic Reviews 2017(11). doi: 10.1002/14651858.CD011767.pub2.
[2] Kozlowski P, Burkhardt T, Gembruch U, Gonser M, Kähler C, Kagan KO, Tercanli S et al. (2019). DEGUM, ÖGUM, SGUM and FMF Germany recommendations for the implementation of first-trimester screening, detailed ultrasound, cell-free DNA screening and diagnostic procedures. Ultraschall in der Medizin, 40(02), 176-193. doi: 10.1055/a-0631-8898.
[2] Vanstone M, Cernat A, Majid U, et al. Perspectives of pregnant people and clinicians on noninvasive prenatal testing: A systematic review and qualitative meta-synthesis. Ontario Health Technology Assessment Series 2019;19(5):1–38. Abrufbar unter: https://www.hqontario.ca/Portals/0/documents/evidence/reports/qualrep-noninvasive-prenatal-testing.pdf (30.11.2020).
[3] BZgA. Genetische Bluttests auf Chromosomen-Abweichungen. Abrufbar unter: https://www.familienplanung.de/schwangerschaft/praenataldiagnostik/bluttests-auf-trisomien/ (30.11.2020).
[4] Schwartz FW (2003). Das Public Health Buch: Gesundheit und Gesundheitswesen: Gesundheit fördern, Krankheit verhindern. Urban und Fischer.
