These fact boxes will help you to weigh the benefits and harms of flexible sigmoidoscopy for the early detection of colon cancer. The information and numbers are based on the best scientific evidence currently available.
The fact boxes were developed by the Harding Center for Risk Literacy.
Colon cancer often entails growths in the mucous membrane of the colon (intestinal polyps). More rarely, cancer occurs in the small intestine. Intestinal polyps are initially benign protrusions of the mucous membrane that protrude into the intestine and often do not cause any symptoms at first. If intestinal polyps become larger, they can occasionally lead to intestinal complaints such as pain or flatulence and to stool irregularities in the form of diarrhea and constipation. They can be harmless, but they can also develop into colon cancer over many years. For that reason, intestinal polyps are also called precancerous colon lesions. They can be monitored or treated and removed through various procedures [1, 2].
In 2018, about 33 per 100,000 women and about 52 per 100,000 men were diagnosed with colon cancer in Germany. People over 55 years of age are most frequently affected [2].
Early detection examinations (also called screening) are intended to detect and treat colorectal cancer and its precursors at an early stage. These examinations are aimed at people who have neither colon cancer symptoms nor a particular risk of colon cancer.
One screening method is flexible sigmoidoscopy. After the colon is partially emptied, for example after a small cleansing enema, the last 60 centimeters of the colon are examined with an endoscope. To get a good view of the colon wall, the colon is slightly inflated during the examination. With the help of the sigmoidoscope, it is then possible to look at the intestinal wall and examine it for abnormalities. In addition, precancerous lesions, the polyps, can be directly assessed and removed if necessary [3].
For the early detection of colon cancer, flexible sigmoidoscopy is not covered by the public health insurance system in Germany. It must be paid for by the patient.
The following alternative screening examinations are covered by public health insurance providers in Germany, graded by age and gender [4]:
- Women and men between 50 and 54 years of age are offered a test for occult blood (hidden blood in the stool) annually and every two years after that.
- Women aged 55 and over and men aged 50 and over are offered a maximum of 2 major colonoscopies (in which the entire colon is examined) at intervals of 10 years. If abnormalities are removed, the examination takes place at shorter intervals.

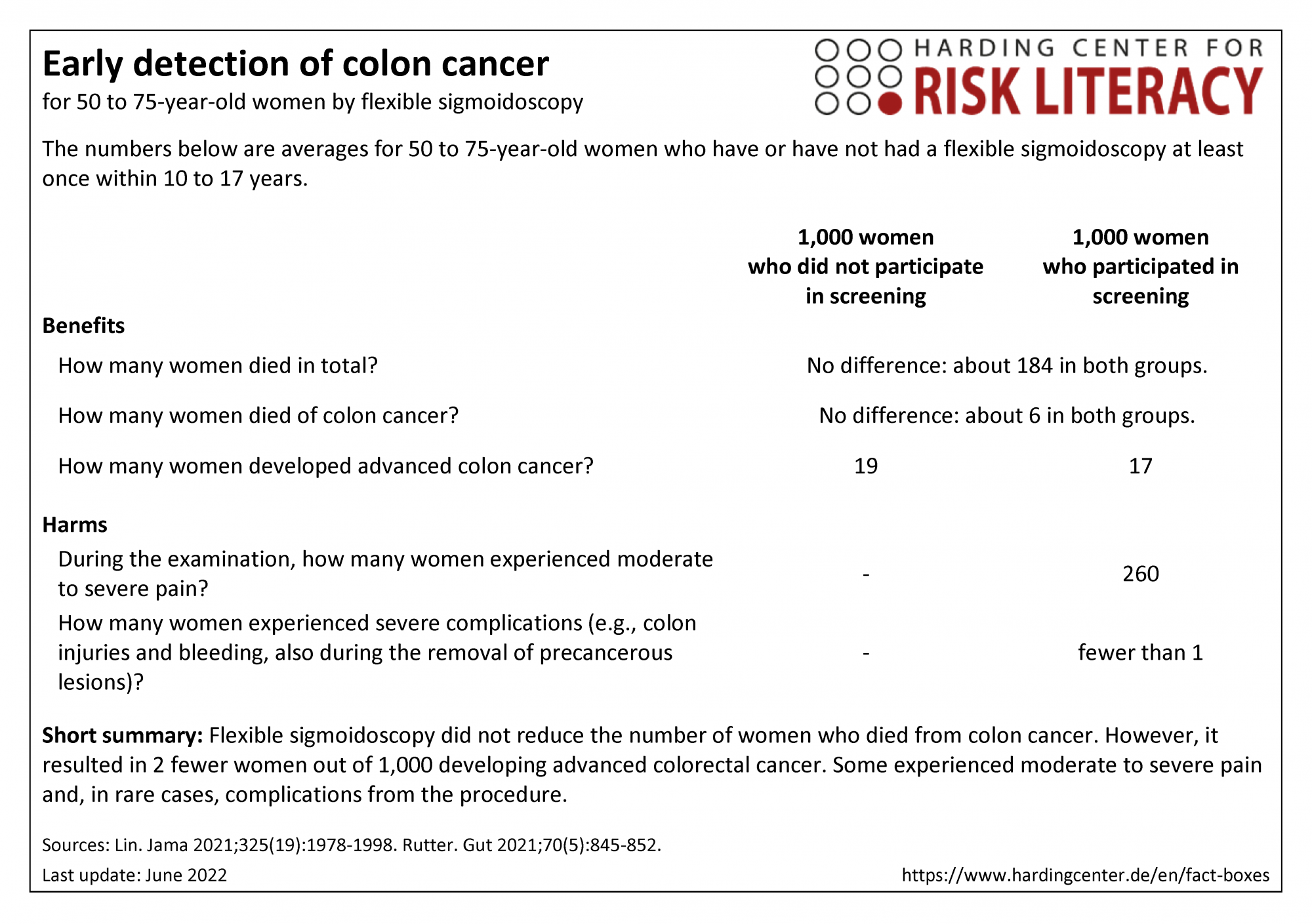

The three fact boxes show the benefits and harms of colon cancer screening by flexible sigmoidoscopy compared with no colon cancer screening. The first fact box shows the benefits and harms of colon cancer screening by flexible sigmoidoscopy compared with no colon cancer screening for women and men aged 50 to 54. The second fact box presents these figures for women aged 50 to 75, and the third fact box for men aged 50 to 75.
The table may be read as follows, using the example of early detection of colon cancer by flexible sigmoidoscopy in women and men aged 50 to 54:
The same number of people aged 50 to 54 died of colorectal cancer within 15 years, independent of whether they participated in screening by flexible sigmoidoscopy or not. In the same time period, 4 fewer persons out of every 1,000 persons who did participate developed advanced colon cancer.
The numbers in all three fact boxes are rounded. The numbers regarding mortality, colon cancer mortality, and diagnoses of advanced colon cancer are calculated from four studies that included a total of around 300,000 participants. These numbers are listed separately in the fact boxes for women, men, and both genders in total.
The numbers regarding complications are calculated from 18 studies that included a total of around 395,000 participants [3].
The numbers regarding pain are calculated from one study that included a total of 1,123 participants [5]. Both harms are given for both genders in total.
A conspicuous finding of intestinal polyps is not a diagnosis of cancer. In some people these intestinal polyps remain harmless; in others they develop over many years into an invasive tumor that can be life-threatening [1].
Overdiagnosis can occur with any screening. In the case of colon cancer screening, this means that colon polyps that grow slowly or not at all might never have caused symptoms. It is difficult for healthcare professionals to judge whether a colon polyp can turn into colon cancer, which is why removal is often advised. This also means that removals are performed unnecessarily, which can lead to complications.
The evidence on benefits and serious complications was determined by the authors of the included review. The overall quality of the evidence across all three fact boxes is moderate to high. [3].
The evidence on pain during the study was determined by the authors of these fact boxes. The evidence across all three fact boxes is of moderate quality [5].
High quality evidence:
We trust the estimates of effectiveness in terms of all-cause mortality, colorectal cancer mortality, and colorectal cancer diagnoses. The actual effectiveness is very likely close to the estimated effectiveness.
Moderate quality of evidence:
We have slightly limited confidence in the estimate for the number of people who suffered from pain during the screening. The actual number of people who suffered pain is likely to be close to the estimate, but is possibly quite different.
-
June 2022 (new search, update of evidence, implementation of new and gender-specific endpoints)
- June 2019 (update of evidence, implementation of gender-specific endpoints)
- June 2016 (creation)
Information within the fact boxes was obtained from the following sources:
[1] Institute for Quality and Efficency in Health Care (IQWiG) (2021). Colorectal cancer. https://www.informedhealth.org/colorectal-cancer.html (26.0.2022).
[2] Robert Koch Institute (RKI). Center for Cancer Registry Data (ZfKD). Cancer in Germany: Colon and rectum 2018 (Stand 29.11.2021). https://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany/cancer_chapters_2017_2018/cancer_c18-c20.pdf?__blob=publicationFile (26.04.2022).
[3] Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR (2021). Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA, 325(19):1978-1997. doi: 10.1001/jama.2021.4417.
[4] Federal Joint Committee (G-BA, Germany). (o. J.). Früherkennung von Krankheiten. https://www.g-ba.de/themen/methodenbewertung/ambulant/frueherkennung-krankheiten/ (28.04.2022).
[5] Rutter MD, Evans R, Hoare Z, Von Wagner C, Deane J, Esmaily S & Beintaris, I. (2021). WASh multicentre randomised controlled trial: water-assisted sigmoidoscopy in English NHS bowel scope screening. Gut, 70(5):845-852. doi: 10. 1136/ gutjnl- 2020- 321918.
Documentation on how the numbers in the fact box were determined is available on request.
